Low cost, high performance, flow battery:
A flow battery is formed by two liquids with opposite charge (electrolytes) which turn chemical energy into electricity by exchanging ions through a membrane. The electrolytes are stored in two external tanks and this makes the system easy to scale up, potentially very quick to charge, and the electrolytes can simply be replaced and resistant to extreme temperatures.
<—more—>
The largest and most stable flow batteries are ones that can be charged thousands of times without suffering degradation or any capacity loss: best possible with vanadium metal. BBC, ViZn, Gildemeister, and ZnPoly all reported that vanadium can lose or gain electrons very easily and by exploiting this property, energy storage technologies advanced.
Imergy Power Systems is a developer of vanadium flow batteries that introduced this batteries first to the market. To date ESP 250 series is the largest line of flow battery from Imergy, fit to operate for 20 years or more (with no need to change or replacement of electrolytes), capable to deliver 250 kilowatts of electrical power for four or more hours. ESP 250 series is ideal for utilities, large commercial, industrial and government end-users, and renewable energy projects. The batteries are more efficient and more cost-effective to meet peak electricity demand. ESP smaller-scale batteries are ESP5 and ESP30 vanadium-flow battery systems, an integrated, ‘plug-and-play’ platform.
Chemistry of redox flow battery: Vanadium chemistry of which oxidized vanadium in sulfuric acid— vanadium is stripped of its five outermost electrons–turns to a yellow solution. Through gradual change in color from green, blue and violet in the presence of zinc, the color change represents electrons being passed to the vanadium. Vanadium-reaction

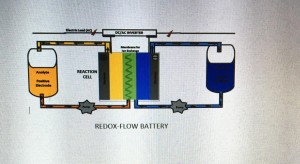
Vanadium Redox Flow Battery
- It consists of two giant tanks of different solutions of Vanadium separated by a membrane. Basic points of Redox-Fow-Battery
- Electric current generates as the fluids in the battery are pumped on either side of the electrodes.
- Vanadium releases electrons in one tank turning the solution color to yellow.
- On the other tank, Vanadium receives electrons turning the solution color from blue to green to violet.
- Electrons generates the current. At the same time matching number of protons (H+ ions) pass across the membrane between the solutions.
Overall, vanadium battery chemistry is very simple. The additional properties to consider here are: vanadium’s long life and it is recyclable. Vanadium can be charged and discharged 20,000 times without loss of performance–about ten times that of Li-ion batteries. The battery is nontoxic, safer and reliable and it lasts for decades–
Downsides of flow battery:
i) Cost- V is not expensive, however refining V is high costs
ii) Huge size- only large scale projects fit in the design. What the vanadium system cannot do is be reduced in size.
iii) Is not suitable for Electric cars or even garage, but effectively used by electrical utilities and other large-end users with solar will be better. V- Batteries can hold enough energy to supply a street with enough power for its TVs, ovens and electric cars every night.
iv) More storage can be achieved by adding just larger tanks of electrolyte.
v) Redox-flow batteries possesses only a third the energy density of a lithium battery.
X-X

good post.Ne’er knew this, regards for letting me know.
Some truly fantastic content on this site, regards for contribution.
Great wordpress blog here.. It’s hard to find quality writing like yours these days. I really appreciate people like you! take care
Where there is a will, there is a way.
Hello! I just would like to give a huge thumbs up for the great info you have here on this post. I will be coming back to your blog for more soon.
Nice post. I used to be checking constantly this weblog and I’m inspired!
Extremely useful information specifically the last part
🙂 I take care of such information much. I used to be looking
for this particular info for a long time. Thank
you and good luck.
GSA Search Engine Ranker Help
gsa search engine ranker backlinks
I like this site very much, Its a real nice post to read and get info . “The love of nature is consolation against failure.” by Berthe Morisot.
Greetings! I’ve been reading your blog for a long time now and finally got the bravery to go ahead and give you a shout out from Houston Tx! Just wanted to tell you keep up the fantastic job!
Very interesting points you have remarked, appreciate it for posting.
Deference to website author, some excellent entropy.
Some genuinely nice stuff on this site, I love it.
Wow! This can be one particular of the most useful blogs We’ve ever arrive across on this subject. Actually Magnificent. I am also a specialist in this topic therefore I can understand your effort.
Hey there would you mind stating which blog platform you’re using? I’m going to start my own blog soon but I’m having a difficult time choosing between BlogEngine/Wordpress/B2evolution and Drupal. The reason I ask is because your layout seems different then most blogs and I’m looking for something unique. P.S Apologies for being off-topic but I had to ask!
Hello
To answer your question: I do not know anything.
I hired professional web-designers, and they developed my web page.
OfCourse, page images I provided.
Thank you for asking.
Fouzia, Author of the weblink
Its good as your other articles : D, regards for posting.
I will right away grasp your rss as I can’t find your e-mail subscription hyperlink or newsletter service. Do you have any? Kindly let me know in order that I may subscribe. Thanks.
I am continuously looking online for ideas that can assist me. Thank you!
Wow! This can be one particular of the most beneficial blogs We’ve ever arrive across on this subject. Actually Fantastic. I’m also an expert in this topic so I can understand your effort.
Excellent read, I just passed this onto a friend who was doing some research on that. And he actually bought me lunch because I found it for him smile Therefore let me rephrase that: Thank you for lunch! “Remember It is 10 times harder to command the ear than to catch the eye.” by Duncan Maxwell Anderson.
A person essentially lend a hand to make critically articles I’d state. This is the very first time I frequented your website page and so far? I surprised with the analysis you made to make this actual put up amazing. Fantastic job!
Perfectly composed content material, Really enjoyed reading.
It is appropriate time to make some plans for the future and it’s time to be happy. I have read this post and if I could I want to suggest you some interesting things or suggestions. Perhaps you can write next articles referring to this article. I desire to read more things about it!
I genuinely enjoy looking at on this web site, it has wonderful blog posts. “The living is a species of the dead and not a very attractive one.” by Friedrich Wilhelm Nietzsche.
As a Newbie, I am constantly searching online for articles that can benefit me. Thank you
Fantastic site. Plenty of helpful info here. I¦m sending it to several buddies ans also sharing in delicious. And of course, thanks for your effort!
My brother recommended I might like this web site. He was entirely right. This put up actually made my day. You cann’t believe just how so much time I had spent for this information! Thank you!
Admiring the time and effort you put into your website and in depth information you offer. It’s good to come across a blog every once in a while that isn’t the same outdated rehashed material. Fantastic read! I’ve bookmarked your site and I’m including your RSS feeds to my Google account.
I like this web site so much, saved to fav.
I was very pleased to find this web-site.I wanted to thanks for your time for this wonderful read!! I definitely enjoying every little bit of it and I have you bookmarked to check out new stuff you blog post.
Hey! Someone in my Facebook group shared this website with us so I came to take a look. I’m definitely loving the information. I’m book-marking and will be tweeting this to my followers! Wonderful blog and wonderful design and style.
Admiring the time and energy you put into your website and detailed information you offer. It’s awesome to come across a blog every once in a while that isn’t the same unwanted rehashed information. Wonderful read! I’ve bookmarked your site and I’m adding your RSS feeds to my Google account.
I just wanted to type a brief message so as to thank you for the marvelous guides you are sharing on this website. My considerable internet search has at the end of the day been rewarded with reasonable information to exchange with my close friends. I would suppose that many of us readers are undeniably blessed to be in a really good network with so many outstanding professionals with valuable suggestions. I feel very much blessed to have discovered the website and look forward to plenty of more entertaining times reading here. Thank you again for all the details.